Abstract
Background: Olutasidenib is a potent, selective, oral, small molecule inhibitor of mutant IDH1 (mIDH1). Olutasidenib has previously shown clinical activity in high-risk AML patients (pts) in a Phase 1 clinical trial (Watts, Blood 2019). The planned interim analysis of an ongoing Phase 2 clinical trial (NCT02719574) in R/R mIDH1 AML pts receiving single-agent olutasidenib 150 mg twice-daily showed an overall response rate (ORR) of 46%, including 33% of pts with CR/CRh (de Botton et al., ASCO/EHA 2021). Here we present data analysis on the mutational characteristics of these pts and the relationship between mutations and clinical response.
Methods: The Efficacy Evaluable (EE) set comprised mIDH1R132X pts whose first dose was ≥180 days before the data cut-off (18-JUN-20). The primary endpoint was CR/CRh (complete remission [CR] according to modified IWG 2003 criteria plus CR with partial hematologic recovery [CRh]) response rate. CRh was defined as bone marrow blasts <5%, absolute neutrophil count >0.5×10 9/L, and platelet count >50×10 9/L. ORR, a secondary endpoint, comprised CR+CRh+CR with incomplete recovery (CRi) + morphologic leukemia-free state (MLFS) + partial remission (PR). IDH1 mutation subtypes were determined by central analysis, co-mutations were reported by investigators. Baseline characteristics and mutation subtypes were tabulated for the safety population and analysis of response rate by mutation type was performed on the EE set.
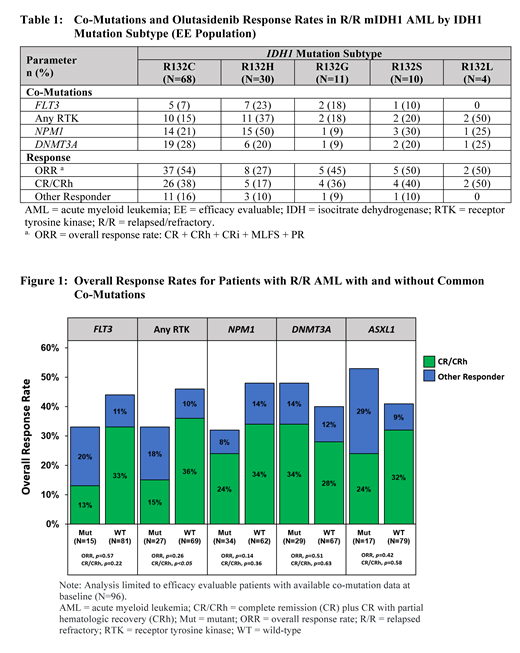
Results: There were 153 R/R AML pts treated with olutasidenib 150 mg BID (safety set). Of those, 123 were in the efficacy evaluable (EE) set (centrally confirmed IDH1R132 mutation and received first dose at least 180 days prior to the data cut off). For the safety population, cytogenetic risk classification was favorable in 6 (4%) pts, intermediate in 109 (71%) pts, and poor in 25 (16%) pts (unknown, 13 [8%] pts). Eighty-five (56%) pts had IDH1R132C mutation subtype, followed by IDH1R132H (n=35 [23%]), IDH1R132G (n=12 [8%]), IDH1R132S (n=11 [7%]), and IDH1R132L (n=4 [3%]). Ninety-four (61%) pts had 1-3 co-mutations reported by the investigator at baseline, with 4-7 co-mutations in 20 (13%) pts, none in 6 (4%) pts, and not done/unknown in 33 (22%) pts. The most common co-mutations at baseline were: NPM1 (n=40 [26%]), DNMT3A (n=36 [24%]), and ASXL1 (n=21 [14%]. Receptor tyrosine kinase (RTK) mutations were reported in 32 (21%) pts (FLT3, n=18 [12%]; NRAS, n=10 [7%]; KRAS, n=3 [2%]; PTPN11, n=3 [2%]; KIT, n=2 [1%]; NF1, n=2 [1%]), with multiple mutations reported in some pts.
For the EE population, responses were achieved in all IDH1R132 mutation subtypes, with ORR and CR/CRh response rates ranging from 27%-54% and 17%-50%, respectively (Table 1). The CR/CRh response rate was lower for pts with IDH1R132H mutations; notably, these pts tended to have a higher percentage of co-mutations, particularly mutations in NPM1 and RTK genes, including FLT3. For EE pts with available co-mutation data (n=96), the mean (SD) number of co-mutations was lower (p<0.05) in pts achieving CR/CRh (1.8 [1.1]; n=29) compared to pts with non-CR/CRh responses or non-responders (2.5 [1.7]; n=67). The ORR was 32%, 48%, and 53% for pts with NPM1, DNMT3A, and ASXL1 mutations, respectively, with corresponding CR/CRh rates of 24%, 34%, and 24% (Figure 1). Pts with RTK mutations at baseline had an ORR of 33% as compared to 46% for pts without any RTK mutation (p=0.26), with fewer pts with RTK mutations achieving CR/CRh than those without any RTK mutation (15% vs. 36%, respectively [p<0.05]).
Conclusions: Responses were observed across IDH1R132 mutation subtypes, with a relatively lower CR/CRh response rate for pts with a R132H mutation as compared to other subtypes. Pts with a best response of CR/CRh had fewer co-mutations than pts who did not achieve CR/CRh. While the ORR was not significantly reduced for pts with RTK mutations, these pts had a lower CR/CRh response rate compared to pts without any RTK mutations. Additional genetic analyses using ddPCR for IDH1 mutations and NGS on a targeted panel of genes at baseline, best response, and end of study will be presented to further explore primary and secondary resistance mechanisms.
De Botton: Celgene, Agios, Forma Therapeutics, Astella, Daiichi Sankyo, Syros, Abbvie, Bayer, Seattle Genetics, Janssen: Honoraria; Celgene, Agios, Astellas, Daiichi Sankyo, Syros, Abbvie, Bayer, Janssen, Pierre Fabre, Novartis, Pfizer, Servier: Consultancy; Celgene: Speakers Bureau; Agios, Forma Therapeutics: Research Funding. Yee: Forma Therapeutics: Research Funding; Onconova: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MedImmune: Research Funding; Jazz: Research Funding; Tolero: Research Funding; AbbVie: Honoraria; F. Hoffmann La Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Geron: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; TaiHo: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Paladin: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb/Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding. Recher: BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; Janssen: Honoraria; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MaatPharma: Research Funding; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wei: Gilead: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Macrogenics: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Montesinos: Forma Therapeutics: Consultancy; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Tolero Pharmaceutical: Consultancy; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Glycomimetics: Consultancy; Stemline/Menarini: Consultancy; Agios: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau. Pigneux: Amgen: Consultancy; Sunesis: Consultancy, Research Funding; BMS Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Braun: Servier: Research Funding; Daiichi-Sankyo, Celgene: Consultancy, Honoraria. Curti: Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Esteve: Novartis: Consultancy, Research Funding; Jazz: Consultancy; Pfizer: Consultancy; Astellas: Consultancy; Novartis: Research Funding; Bristol Myers Squibb/Celgene: Consultancy; Abbvie: Consultancy. Grove: Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Jonas: 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo Oncology, Pfizer, Pharmacyclics, Sigma Tau, Treadwell: Research Funding; AbbVie, BMS, Genentech, GlycoMimetics, Jazz, Pfizer, Takeda, Treadwell: Consultancy; AbbVie: Other: Travel reimbursement. Khwaja: Pfizer, Abbvie: Honoraria; Novartis, Jazz: Speakers Bureau. Blum: Forma Therapeutics, Xencor; Celyad: Research Funding; Amerisource Bergen; Abbvie, Syndax: Honoraria. Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Jurcic: AbbVie, BMS/Celgene, Novartis: Consultancy; AbbVie, Arog Pharmaceuticals, Astellas, BMS/Celgene, Forma Therapeutics, Genentech, Gilead Sciences, PTC Therapeutics, Syros Pharmaceuticals: Research Funding. Watts: Rafael Pharmaceuticals: Consultancy; Genentech: Consultancy; Bristol Myers Squibb: Consultancy; Takeda: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Aptevo Therapeutices: Research Funding. Xin: Forma Therapeutics, Inc.: Current Employment. Sedkov: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Guichard: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sweeney: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Cortes: Sun Pharma: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Fenaux: Syros Pharmaceuticals: Honoraria; JAZZ: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal